Embarking on an exploration of charged in chemistry crossword clue, this discourse unveils the fundamental principles governing electric charge within chemical systems. Delving into the very nature of charge, we elucidate its significance in shaping the behavior of atoms, molecules, and ions, ultimately dictating the course of chemical reactions and a myriad of practical applications.
Our journey begins with a thorough examination of ion formation and charge, unraveling the intricate mechanisms by which atoms acquire or shed electrons, transforming into charged species. We delve into the concept of ionic bonding, exploring its role in the formation of ionic compounds and their distinctive properties.
Charged in Chemistry: Charged In Chemistry Crossword Clue
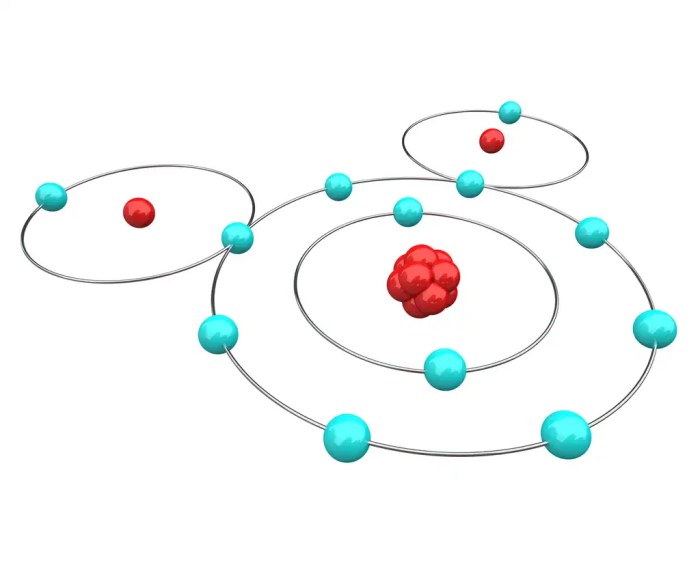
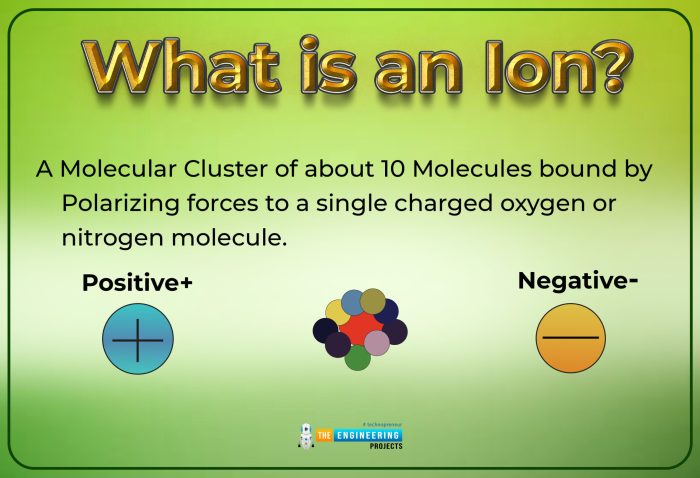
Electric charge is a fundamental property of matter that arises from the presence of charged particles, such as electrons and protons. In chemistry, the term “charged” refers to the presence of an imbalance between the number of electrons and protons in an atom or molecule, resulting in a net positive or negative charge.
Ion Formation and Charge
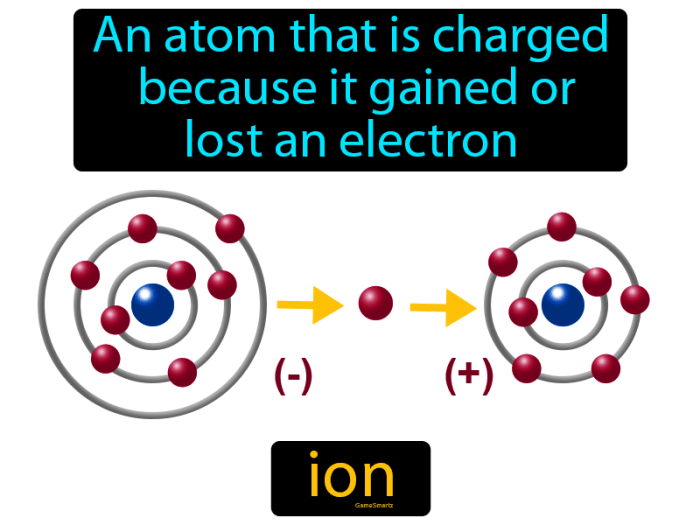
Ion formation occurs when an atom or molecule gains or loses electrons, resulting in a change in its charge. When an atom loses electrons, it becomes positively charged and is known as a cation. Conversely, when an atom gains electrons, it becomes negatively charged and is known as an anion.
The formation of ions is crucial in ionic bonding, where oppositely charged ions are attracted to each other to form ionic compounds.
Charge in Chemical Reactions
Charge plays a significant role in chemical reactions, particularly in redox reactions and acid-base reactions. In redox reactions, electrons are transferred between reactants, resulting in changes in their oxidation states and charges. In acid-base reactions, protons (H +ions) are transferred between reactants, leading to the formation of conjugate acid-base pairs.
Charge Measurement and Characterization, Charged in chemistry crossword clue
Various techniques are used to measure electric charge in chemistry, including potentiometry and conductometry. Potentiometry involves measuring the electrical potential difference between two electrodes to determine the concentration of ions in a solution. Conductometry measures the electrical conductivity of a solution to determine the presence and concentration of ions.
Applications of Charged Species
Charged species have numerous practical applications in various fields. In batteries, charged ions are used as electrolytes to facilitate the flow of electrons. In fuel cells, charged species are involved in the electrochemical reactions that generate electricity. In electroplating, charged metal ions are deposited on a surface to create a thin layer of metal.
Essential Questionnaire
What is electric charge in chemistry?
Electric charge is a fundamental property of matter that describes the ability of a particle to experience an electric force. In chemistry, charge is associated with the presence of electrons and protons, which carry negative and positive charges, respectively.
How are ions formed?
Ions are formed when atoms gain or lose electrons, resulting in a net electric charge. This process can occur through various mechanisms, such as chemical reactions, ionization, or electrolysis.
What is the significance of charge in chemical reactions?
Charge plays a crucial role in chemical reactions, particularly in redox reactions and acid-base reactions. Charge transfer and exchange during these reactions drive the formation of new chemical species and determine the overall reactivity of the system.