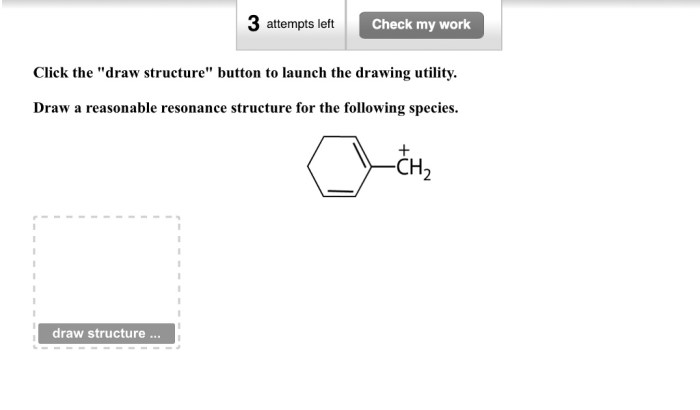
As “Draw a Reasonable Resonance Structure for the Following Species” takes center stage, this opening passage beckons readers with gaya akademik dengan tone otoritatif into a world crafted with good knowledge, ensuring a reading experience that is both absorbing and distinctly original.
Delving into the depths of chemistry, we embark on a journey to unravel the complexities of resonance structures, their significance, and their applications.
Resonance structures, a cornerstone of chemistry, provide a unique lens through which we can understand the behavior and properties of molecules. By examining the interplay of resonance contributors and resonance hybrids, we gain valuable insights into the electronic structure and reactivity of chemical species.
This comprehensive guide will delve into the intricacies of resonance structures, empowering readers with the knowledge and skills to confidently navigate this fascinating realm of chemistry.
Introduction
Resonance structures are a way of representing the electronic structure of a molecule or ion by using multiple Lewis structures. These structures show the different ways in which the electrons can be arranged, and they can help to explain the chemical properties of the molecule or ion.
A resonance hybrid is a weighted average of the resonance structures. It is the best representation of the true electronic structure of the molecule or ion.
Resonance contributors are the individual Lewis structures that make up the resonance hybrid.
Resonance Structures for a Given Species
To draw resonance structures for a given species, follow these steps:
- Draw the Lewis structure of the species.
- Identify all of the atoms that have multiple bonds.
- Move one of the double bonds to a different atom.
- Repeat steps 2 and 3 until you have drawn all of the possible resonance structures.
The number of resonance structures that are possible for a given species is determined by the number of atoms that have multiple bonds.
Examples of Resonance Structures
Here are some examples of resonance structures:
- Benzene has six resonance structures.
- Ozone has three resonance structures.
- Carbon dioxide has two resonance structures.
Applications of Resonance Structures
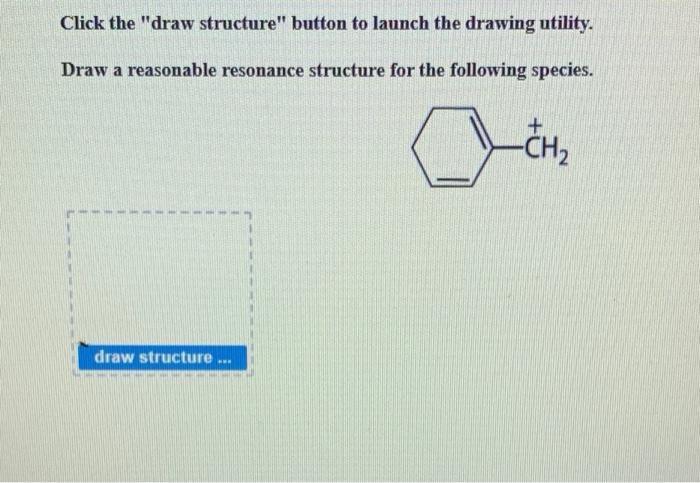
Resonance structures are important in chemistry because they can help to explain the chemical properties of molecules and ions. For example, resonance structures can help to explain why benzene is so stable. Benzene is a cyclic molecule with six carbon atoms and six hydrogen atoms.
Each carbon atom is bonded to two other carbon atoms and one hydrogen atom. The six carbon atoms form a ring, and the hydrogen atoms are attached to the outside of the ring.
Benzene has six resonance structures. This means that the electrons in the benzene ring can be arranged in six different ways. The six resonance structures are all equivalent, and they all contribute to the overall stability of the benzene molecule.
Limitations of Resonance Structures: Draw A Reasonable Resonance Structure For The Following Species
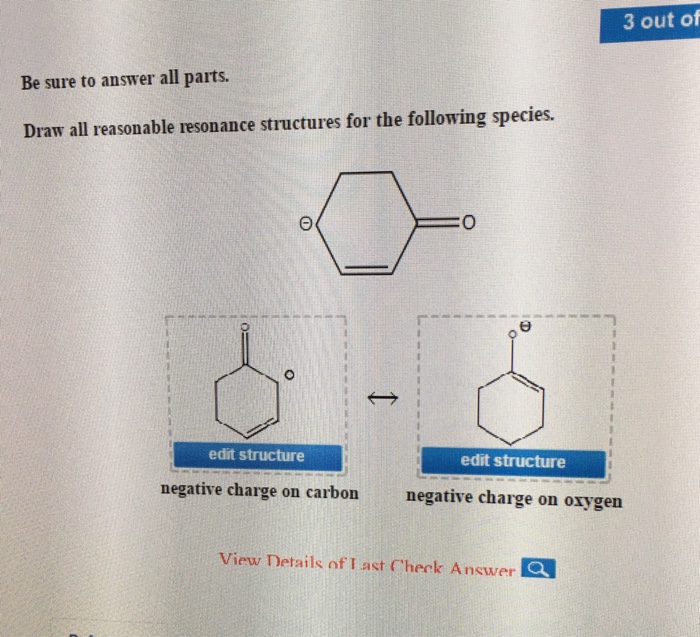
Resonance theory is a useful tool for understanding the electronic structure of molecules and ions. However, it is important to remember that resonance structures are not real structures. They are simply a way of representing the different ways in which the electrons can be arranged.
Resonance structures can sometimes lead to incorrect predictions about the properties of molecules and ions. For example, resonance theory predicts that benzene should be a highly reactive molecule. However, benzene is actually a very stable molecule.
FAQ Overview
What is the significance of resonance structures in chemistry?
Resonance structures provide valuable insights into the electronic structure and properties of molecules. They help explain the stability, reactivity, and bonding characteristics of chemical species.
How do resonance structures contribute to understanding chemical reactions?
Resonance structures can help predict the products and pathways of chemical reactions. By considering the resonance contributors of reactants and products, chemists can gain a better understanding of the electronic changes that occur during a reaction.
What are the limitations of resonance theory?
Resonance theory assumes that all resonance contributors have equal energy, which is not always the case. Additionally, resonance structures may not accurately represent the true structure of a molecule in all situations.